|
Surface Tension is said to act between two immiscible liquids or
between a liquid and gas, say water and air. The interface between
the two fluids is assumed to act like a stretched membrane under
tensile stress. This stress is required to hold the membrane in
position. Many instances from everyday life could be cited to
justify this assumption. A steel needle will float on water due to
the apparent tension developed at the water surface. Water drops
form on smooth surfaces. Tiny spheres of mercury will form when it
is poured on a smooth surface. Unbalanced cohesive forces acting
on liquid molecules at the interface are balanced by the tensile
force due to surface tension.
Surface Tension, 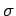 is expressed as force per unit distance
(N/m) and has dimensions of is expressed as force per unit distance
(N/m) and has dimensions of 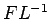 . Its magnitude depends upon
the two fluids in contact and on temperature. . Its magnitude depends upon
the two fluids in contact and on temperature.
Subsections
(c) Aerospace, Mechanical & Mechatronic Engg. 2005
University of Sydney
|

