Density is defined as mass per unit volume of the substance.
However, as discussed before, in the spirit of Continuum
Hypothesis, it is defined as a limit. If 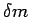 is the mass
of a small volume is the mass
of a small volume
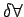 then then
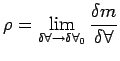 |
(1.13) |
Unit of density in the SI system is 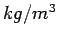 Under ordinary
conditions the density of water is Under ordinary
conditions the density of water is
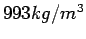 while that for air
at while that for air
at 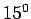 C and atmospheric pressure is C and atmospheric pressure is
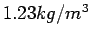 . .
Density of liquids is somewhat insensitive to the changes in
pressure and temperature. For gases there is a strong dependence
of density on these quantities and is given by the equation of
state of the particular gas.
Subsections
(c) Aerospace, Mechanical & Mechatronic Engg. 2005
University of Sydney
|

